AI designed sos1 small molecule inhibitor is effective in kras mutant driven cancer
Siwei Li (1), Yuton Jin (1) , Liang Zhu(1), Yang Jiao(1), Lurong Pan*(1)
Background
- Mutations in the KRAS gene occur in 1/7 of all human cancers. KRAS is most frequently mutated oncogene.
- Activating mutant KRAS lead to aberrant signaling of MAPK pathway. [1-2]
- Most of these point mutations of KRAS alter amino acids G12, G13 or Q61. [2]
- The Son of Sevenless 1 (SOS1) protein is a guanine nucleotide exchange factor (GEF) that facilitates inactive GDP-bound KRAS to active GTP-bound KRAS. [3]
- By preventing the interaction of SOS1 and KRAS, the SOS1 inhibitor blocks reloading of KRAS with GTP, leading to antiproliferative activity. [4]
- Here, we have applied AI technologies in SOS1 small molecule inhibitor design, demonstrate AI capability for small molecule drug design in drug discovery.
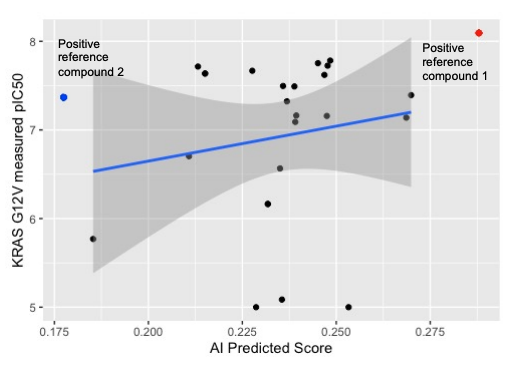
SOS1-KRASG12V binding prediction
- To assess the compounds inhibition on SOS1 and KRAS-G12V binding, 24 AI-designed compounds were tested by KRAS G12V/SOS1 binding assay.
- 66.7% of compounds potency values were less than 42 nM, compared to positive reference compound 2 IC50 range (10-42 nM).
- The experimental measured binding affinity values were correlated with AI binding affinity prediction scores.
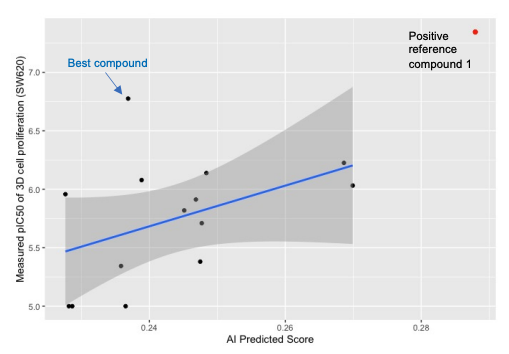
In Vitro anti-proliferation efficacy
- Antiproliferative efficacy of 14 compounds were measured by 3D-cell (SW620, KRASG12V) proliferation assay.
- The measured compound antiproliferation efficacy IC50 values were also correlated with the AI predicted binding affinity scores.
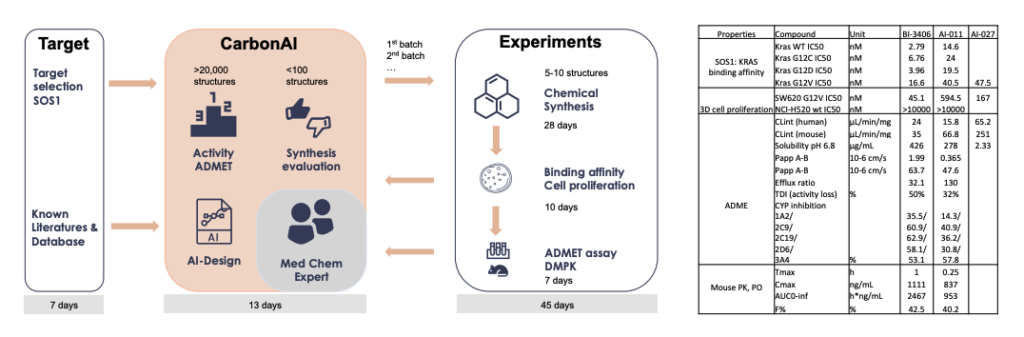
CarbonAI model design, workflow and lead candidates profiles
- The general workflow and timeline for the design of lead candidates using CarbonAI.
- Through AI design, we found a potent and oral bioavailable SOS1 inhibitor AI-011, that prevents KRAS G12C, G12D, G12V activation.
- AI-deisgned SOS1 inhibitors AI-011 and AI-027 have antiproliferative effects in 3D-cell (SW620, G12V) proliferation assay.
- ADME and PK properties of AI-011 were listed in the below table, CYP inhibition and TDI properties of AI-011 were better than the reference compound 1
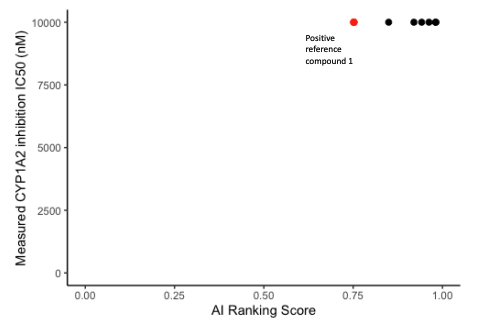
CYP isoform inhibition prediction
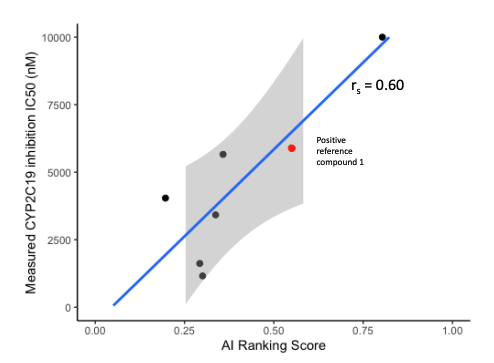
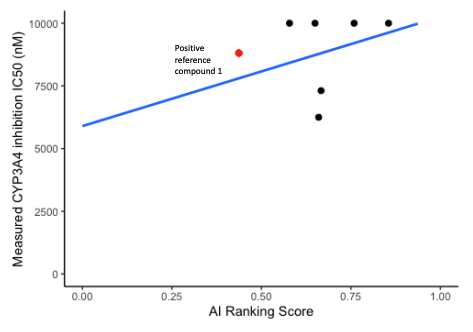
- CYP inhibition of 6 potent compounds were charaterized by in vitro CYP inhibition assay.
- In 6 compounds measurements, our CarbonAI prediction exhibited a good correlation in CYP1A2, CYP2C19 (Spearman’s Rank Correlation Coefficient Rs = 0.60) and CYP3A4 inhibition experimental results.
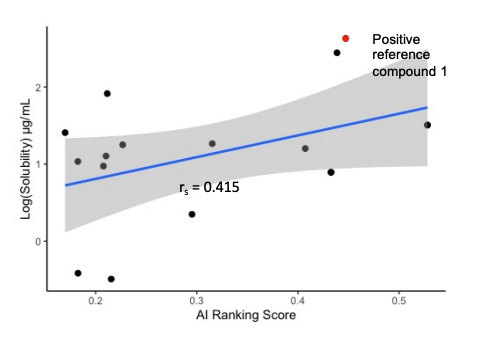
Solubility prediction
A. OD 450 absorbance values obtained by direct ELISA of 50 antibodies (1μg/ml; blue) against wild type (A), delta (B) and Omicron(C) at 1ug/ml. Plate coated with 1ug/ml RBD protein. OD450 = optical density at 450 nm; Two therapeutic SARS-CoV-2 antibodies were tested as controls (black,orange)
Conclusions
- Through AI drug design, we discovered a potent and orally bioavailable SOS1 inhibitor AI-011.
- The AI-design compounds (AI-011, AI-027) were shown antiproliferation efficacy in SW620 (KRASG12V) 3D-cell proliferation assay.
- CarbonAI displays a very good generalization performance in the prediction of SOS1 protein binding affinity, CYP isoforms inhibition (CYP1A2, 2C19 and 3A4) and solubility.
